Red Water

Discoloured Water in Bathurst , NSW , 2013 due to Manganese and Iron
Red water has a long history in domestic drinking water distribution system. It is always associated with problems of water flow restriction, and water quality deterioration. The discolouration is caused by very fine iron, manganese and clay particles that enter the water supply through groundwater bores and stream flow into dams. Sediment forms as pipes, including the fittings and cement lining, get older.One of major source of iron is internal corrosion of grey cast iron which has been utilised as water pipeline materials for long time. The corrosion product (iron oxide) [i]released to water flow is one of the principal causes of ‘‘red water’’ problems in drinking water distribution systems.
There are two relatively independent steps turn potable water into red water:[ii]
- Corrosion of iron is the conversion of “metallic iron” to an oxidized form, either soluble or an oxidized scale.
Fe = Fe(II) + 2e
Fe (II) = Fe(III) + e
- Iron release is the transport of iron, either in soluble form or as a particle, or both, from corrosion scale or metal to bulk water in ferric and/or ferrous forms.[iii]
Corrosion Factors
- DO
It was reported that dissolved oxygen (DO) in pure water would not aggravate the corrosivity in ambient temperature. However, the presence of trace amount of chloride, nitrate and /or sulphate (i.e. 0.5 ppm) depolarise the metal surface, therefore, make oxygenated water highly corrosive.
- pH
In oxygenated drinking water pH range (5~9.5), it appears no significant impact on the cast iron corrosion rate. The corrosion slows down rapidly in higher pH range.
- Temperature
Higher temperature means higher corrosion rate in water distribution system by accelerating corrosion reactions. However there are some other accelerating factors and decelerating factors regarding to temperatures: Excessive temperature (>55oC) means potential reversing of zinc/iron, which turns iron into anode. Intermediate metallic compound (Zn-Fe) formed during hot zinc dipping process is cathodic to both zinc and iron in oxygenated water. In close circulating system, higher oxygen solubility under the pressure means higher corrosion rate in elevated temperature. However, reverse solubility of calcite suggests the scale may inhibit the corrosion by forming a barrier on iron surface.
- Piping materials
Although cast iron materials has long history been used as for potable water distribution networks, the other non-metallic (e.g. Polyethylene (PE), Fibre Reinforced Concert (FBC), etc) and metallic (e.g. Copper, stainless steel etc.) are widely used in the system. From corrosion point, upstream copper/braze components have detrimental effects due to:
- Severe galvanic corrosion due to iron-copper direct connection,
- Cu(II) leaching into water deposits in downstream iron piping surface, which forms new iron-copper couple,
- Copper piping and components is prone to impingement and cavitation in hot water system. The spalled copper particulate will re-settled in downstream of iron piping surface, which forms new galvanic couple,
- The copper is prone to erosion attack at high velocity (1.5m/s). The attack is more severe while solids are entrained.[iv]
- Totals dissolved solids
Conductivity means the ability to transmit electricity. Some waters are more conductive than others. Since corrosion is an electrochemical process, the more conductive the water, the greater the potential for corrosion. Further than that, even trace amount of nitrate, chloride and sulphate will depolarise the iron surface in oxygenate water, thus prompt the corrosion dramatically.
- Microbial effects
Microbiologically influenced corrosion commonly refers to corrosion from iron-related bacteria and sulphate-reducing bacteria. Iron-related bacteria use iron as an energy source. Sulphate-reducing bacteria use sulphate. The significance of bacterial growth is to create a favourable micro corrosive environment, e.g. tubercle cavity and biofilm.
Other factors
- Stagnant
Stagnation increases shell-like layer porosity. Stagnant is favourable for FeOx scale dissolution, which prompts water discolouration.
- Scale oxidisation
Iron release is reduced by scale oxidisation, therefore free chlorine and DO will keep oxidants next to pipe wall and reduce the water discolouration.
Chemical Inhibitors
- Carbonate scaling
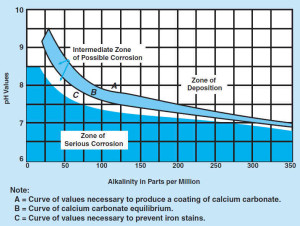
Hardness, alkalinity will prompt scaling, which tends to slow down the corrosion. The uniform, thin and high dense texture coating is much preferred in corrosion control than porous scale[v]. It is only a feasible technical solution at lower pH range (7.0~7.5). This is due to anodic area under higher pH range become larger in area and smaller in numbers, which prompts the deposition of spongy precipitation. Therefore, carbonate is not an effective inhibitor for corrosion and red water in higher pH range. Ferric oxide can act as nucleus and catalyst for deposition of calcite, which is favourable as corrosion inhibition.
- Silicate
Silicate is effective on ferrous and nonferrous metals (lead, copper, yellow brass, aluminium etc.) for corrosion inhibition in potable water system[vi]. Naturally occurrence of silica is usually not active in corrosion inhibition, therefore should not be compensated in dosage rate. Moderately alkaline range (pH) is preferred for silicate inhibition.
- Polyphosphate
Prevention of red water is one of the major objectives of internal corrosion in potable water distribution system. The preferred pH ranging from 5.0-7.0 The Polyphosphate serves dual purpose in the system:
- Inhibit corrosion therefore preventing the Fe(II) pickup,
- Stabilise dissolved Fe(II) in water.
However, polyphosphate can stabilise calcite to prevent the calcite scaling, which made calcite inhibition loss its desired function,
- Polyphosphate Zinc
Polyphosphate zinc improves the efficacy of corrosion inhibition and protective film repair.
Chemical Inhibition Practices
Mechanical pigging is the common practice to restore cast iron pipeline carry capacity. However, red wate problems can be extremely severe without proper engineering control. Higher chemical dosage is usually preferred immediately after the pigging to restore the protective film. Higher concentration of polyphosphate assists the dispersion of settlement. The chemical dosage rate gradually drops to normal rate within months after the cleaning pigging.
In small scale system, the water passes through a slowly soluble phosphate glass bed to pick up the polyphosphate.
For zinc and polyphosphate dosing, the chemicals can be introduced separately as a soluble salt. The optimum range for each is somehow interdependent: higher Zinc levels permit lower polyphosphate and vice versa. Zinc is usually 10% of polyphosphate feed. Normally, rapid soluble zinc phosphate compounds (e.g. Na2O∙ZnO∙P2O5) are utilised for easy dosage.
Reference
[i].Sarin P, Snoeyink VL, Bebee J, Jim KK, Beckett MA, Kriven WM, Clement JA. Iron release from corroded iron pipes in drinking water distribution systems: effect of dissolved oxygen. Water Res. 2004 Mar;38(5):1259-69
[ii] Xiaojian Zhang, Zilong Mi, Yang Wang, Shuming Liu, Zhangbin Niu, Pinpin Lu, Jun Wang, Junnong Gu, Chao Chen A red water occurrence in drinking water distribution systems caused by changes in water source in Beijing, China: mechanism analysis and control measures Frontiers of Environmental Science & Engineering June 2014, Volume 8, Issue 3, pp 417-426
[iii] Lytle*, D A. AND V. L. Snoeyink. DRINKING WATER QUALITY DETERIORATION IN DISTRIBUTION SYSTEMS: COLORED WATER FORMATION AND ITS CONTROL. Presented at 2003 AWWA Distribution Systems Symposium Meeting, Portland, OR, 09/28/2003.
[iv] http://www.copper.org/applications/plumbing/techcorner/designing_piping_systems.html
[v] Faculty.washington.edu, Causes and Cures of Distribution System Corrosion
[vi] www.pqcorp.com Sodium Silicate Corrosion Inhibitors: Issues of Effectiveness and Mechanism




Pingback: Corrosion of ASTM 304 Grade Stainless Steel for Drinking Water Service - Corrosion and Corrosion Control
link