Electrochemical metal corrosion reactions are expressed as controlled by anodic reaction, cathodic reaction, or both. The Tafel slope β is obtained by plotting the log of applied current, ip versus the reference potential, η. These slopes can be measured for the cathodic polarisation βc and anodic polarisation βa :
η = a + b log ip
in which, a is an empirical constant; b is the slope
d η / d log ip = β
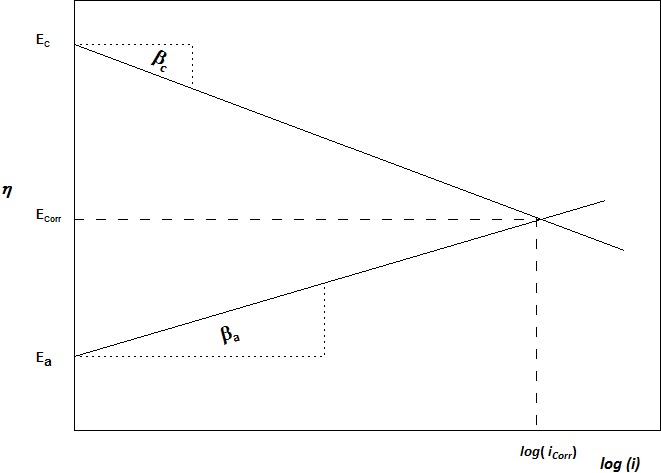 When a metal is in corrosion balance with surrounding electrolyte, the anodic currents (ia) and cathodic currents (ic) are equal and no net reaction. If the equilibrium is shifted by whatever reasons, the metal surface will be polarised either cathodically or anodically. For cathodic polarisation, the electrons flow away from metal, i.e. anodic current is evident. While for anodic polarisation, the electrons push into metal surface.
When a metal is in corrosion balance with surrounding electrolyte, the anodic currents (ia) and cathodic currents (ic) are equal and no net reaction. If the equilibrium is shifted by whatever reasons, the metal surface will be polarised either cathodically or anodically. For cathodic polarisation, the electrons flow away from metal, i.e. anodic current is evident. While for anodic polarisation, the electrons push into metal surface.
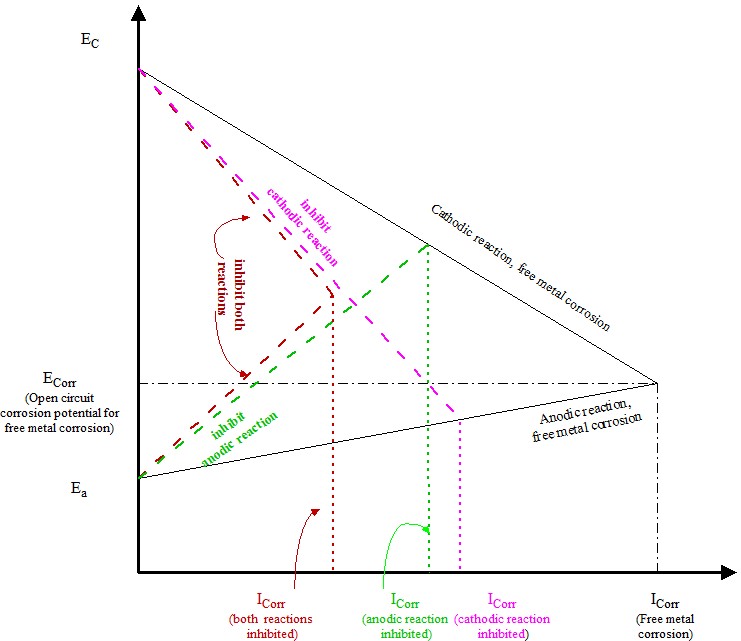
The corrosion inhibition is to use corrosion inhibitor to influence the corrosion reaction. The free metal corrosion and inhibited metal corrosion reactions are shown in the following Tafel diagram. The point of intersection of anodic reaction and cathodic reaction is the open circuit corrosion potential of the metal in such corroding environment. The corrosion currents are proportional to metal corrosion rate.
 The corrosion current can be derived by Stern equation:
The corrosion current can be derived by Stern equation:
in which, Icorr is corrosion current; R is polarisation resistance. Corrosion current, i.e. corrosion rate, is proportional to inversely of the polarisation resistance.
The cathodic site adsorbers are generally Proton Acceptors. Organic inhibitors such as anilines, quinolines, ureas and aliphatic amines, are used in acidic environment as cathodic reaction inhibitor.
Anodic reaction inhibitors are normally Electron Acceptors. They are most effective to inhibit the corrosion reaction under anodic control.
Passivating inhibitors are normally Electron Acceptors as well: organic peroxides, organic thiols, selenols and chromates and nitrite.
Ambiodic Inhibitors have ability to affect both anodic and cathodic reactions, which are most likely to have high corrosion inhibition efficiency

New to your blog. Stumbled upon it browsing the web. Keep up the great work. I am hoping you update it regularly.